

Ĥ)They are poor conductors of heat and electricity.This is due to lack of the free electrons in their structure.Thus, they act as good insulators of heat and electricity.ĥ)Solid non metals are brittle. Most of them,which are solids, are dull in appearance. Gas – Chlorine,Oxygen,All noble gases (Helium,Argon,Neon,Xenon etc)ģ)They don’t have metallic lustre. They have strikingly different properties than the metals and this is mainly due to their electronic structure.! 17 elements are classified as non metals -Hydrogen,helium, bromine,noble gases,fluorine,chlorine,nitrogen,oxygen,carbon,phosphorous,sulphur,iodine,selenium.ġ)They generally have 4-8 electrons in their valence shell.Thus, they have high ionization energies i.e energy required to remove the valence electron to form a cation.Non metals, therefore, gain or share electrons.They thus form anions in ionic reactions.Ģ)They can be solid,liquid or gaseous at room temperature. Non-metals are elements, which are found in nature, which do NOT show properties like metals.
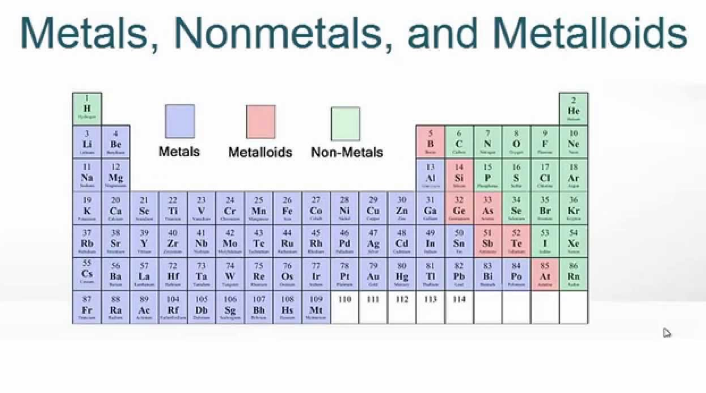
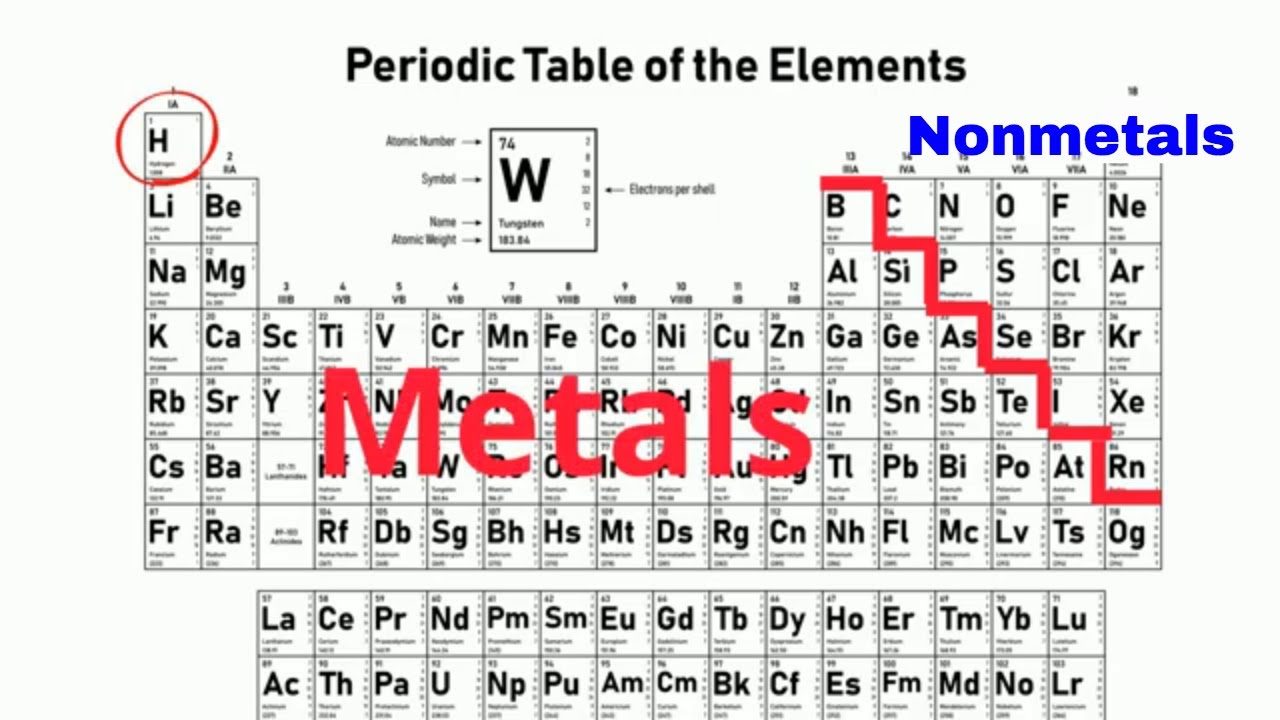
Let us begin our discourse on the remaining elements of the periodic table. In our last post we discussed the properties of metals in detail.In this post, let us look into non-metals and metalloids.Let us figure out, how these two can be differentiated from metals.


 0 kommentar(er)
0 kommentar(er)
